

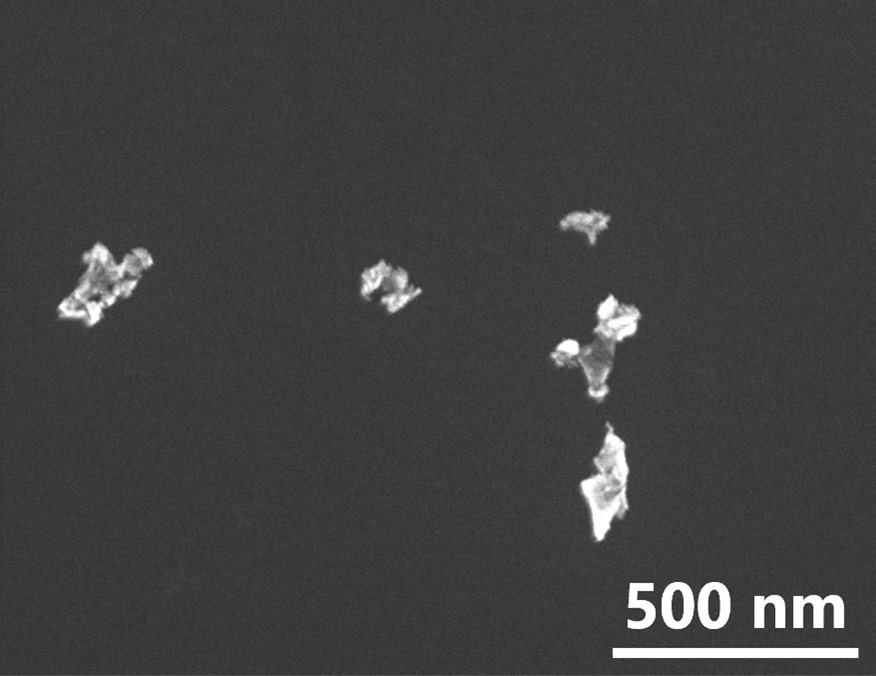
In recent years, there has been a growing focus on the application of diamonds with NV (Nitrogen-Vacancy) centers, particularly in the field of biotechnology. The NV center is a lattice defect consisting of nitrogen (N) and atomic vacancies (V) and exhibits three charge states (NV+/NV0/NV-), each emitting different colors of fluorescence depending on their charge state. Fluorescent nanodiamonds containing these NV centers hold promise for local measurements. Furthermore, their fluorescence is advantageous as a fluorescent labeling material since it does not fade or blink at room temperature or in solution. Additionally, due to the long electron spin coherence time of NV-, applications in quantum measurements using NV centers are actively pursued. However, NV centers near the surface are susceptible to the influence of surface defects and adsorbents, leading to unstable charge states, which has prompted the need to improve charge stability.
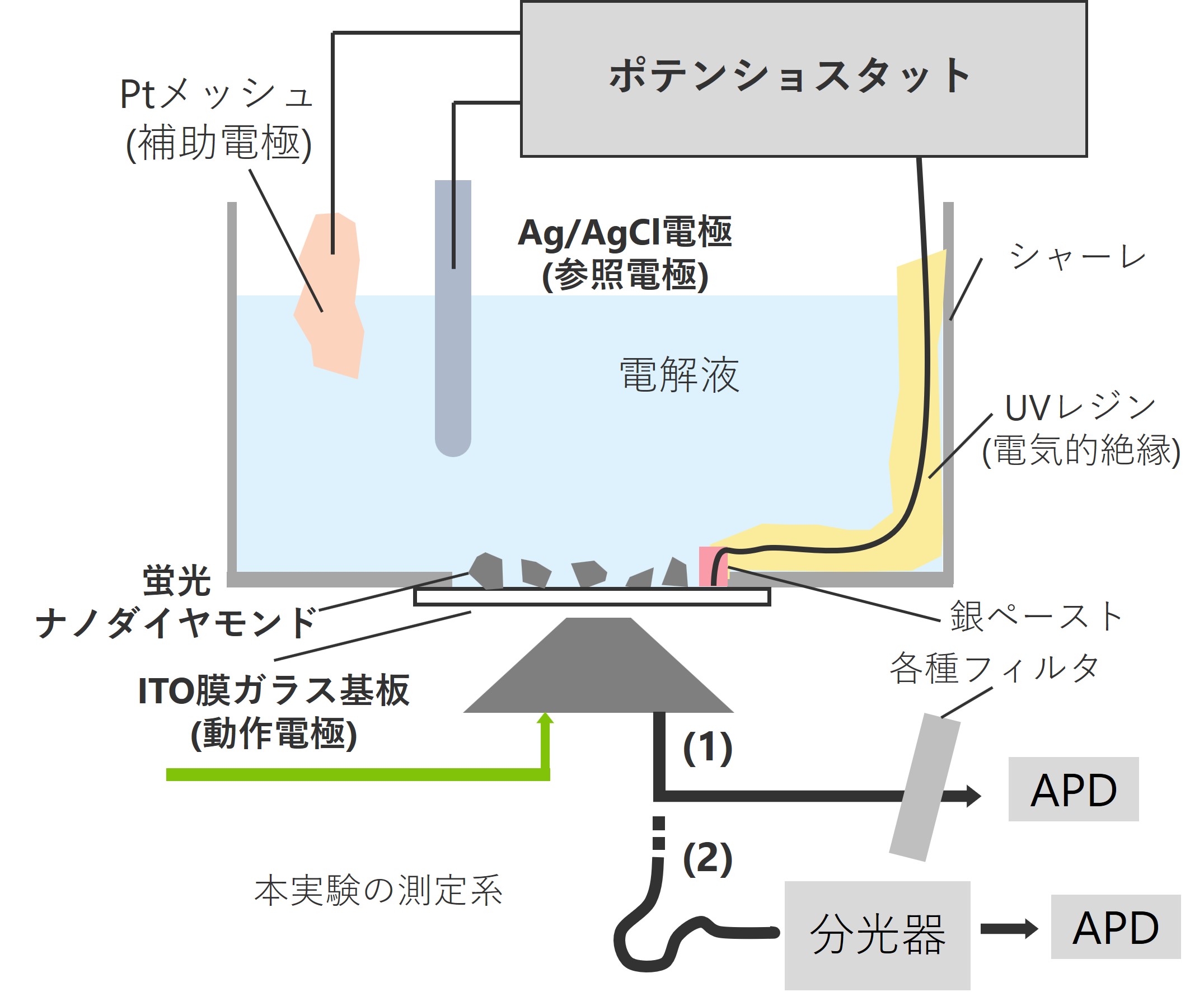
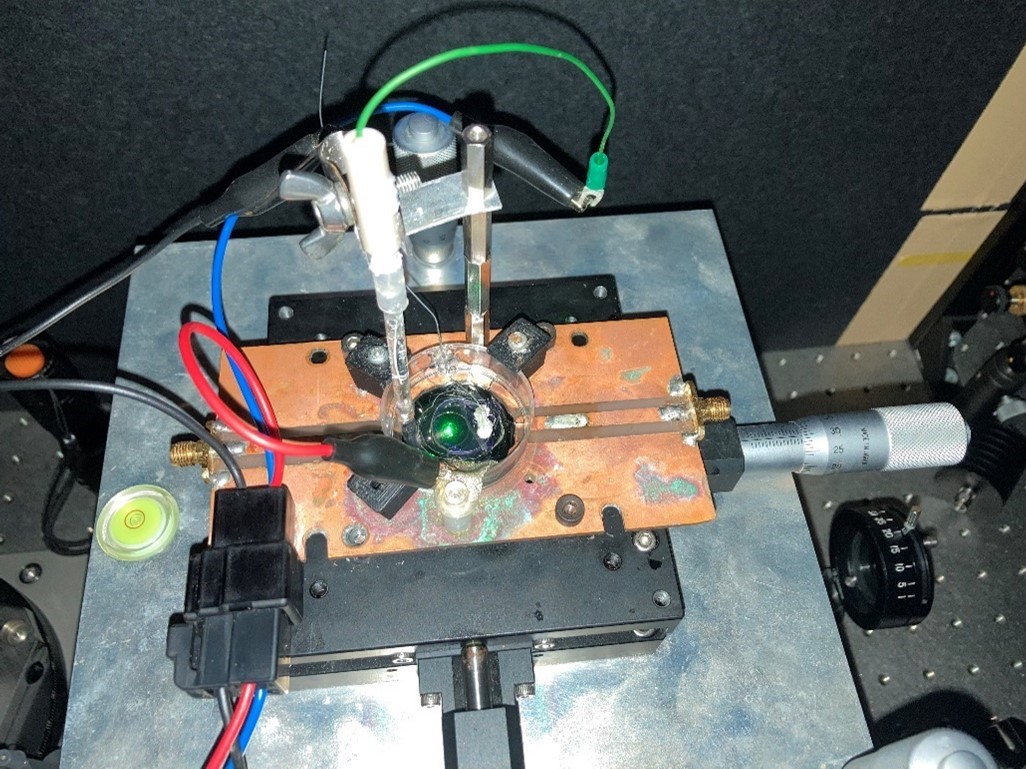
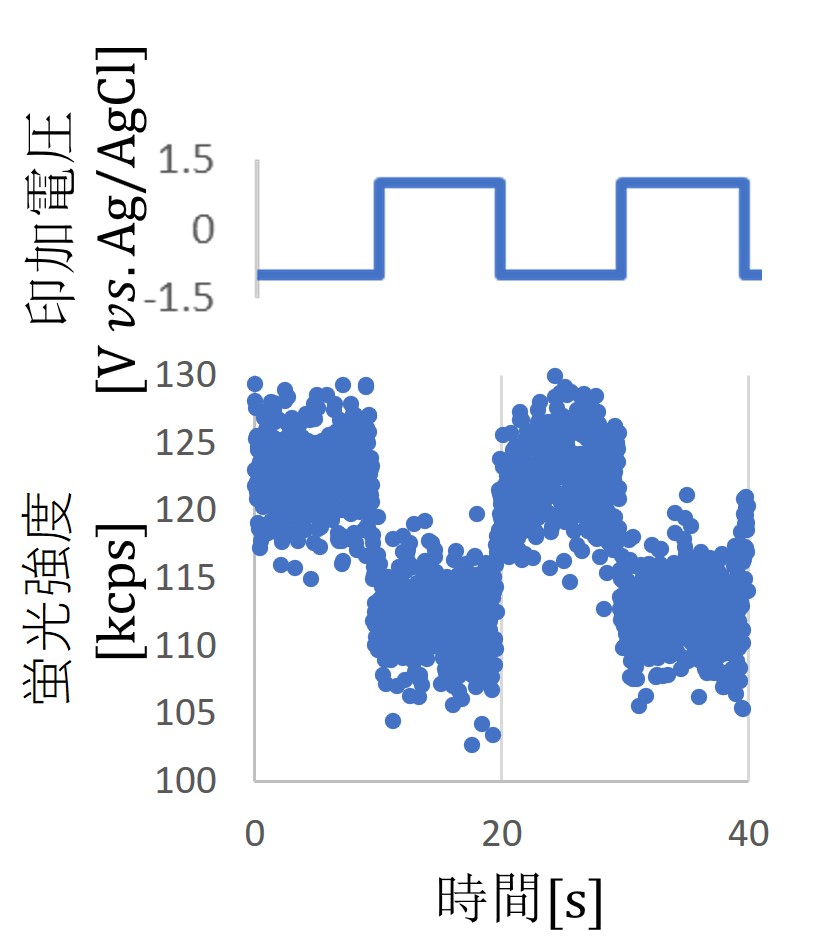
We believe that the inherent instability of these charge states can be actively utilized to create local sensors that respond to changes in the external environment. To this end, we have successfully used an electrochemical cell to apply square wave voltages and observed changes in charge states using a confocal laser scanning microscope available in our laboratory. We have investigated how the response ratio and sensitivity of NV centers to changes in potential vary with respect to the termination and particle size of nanodiamond surfaces and the superposition of DC biases.
Currently, we are conducting research to understand the mechanism by which charge states change in NV centers within nanodiamonds. If we can provide a detailed explanation of how charge states change in response to changes in potential, it is believed that this will contribute to improving charge stability in electron spin manipulation and measurement, enabling fluorescent nanodiamonds to function as nano quantum sensors capable of measuring magnetic fields, electric fields, and temperature on a nanoscale.